SUBMUCOSAL INJECTION AGENT
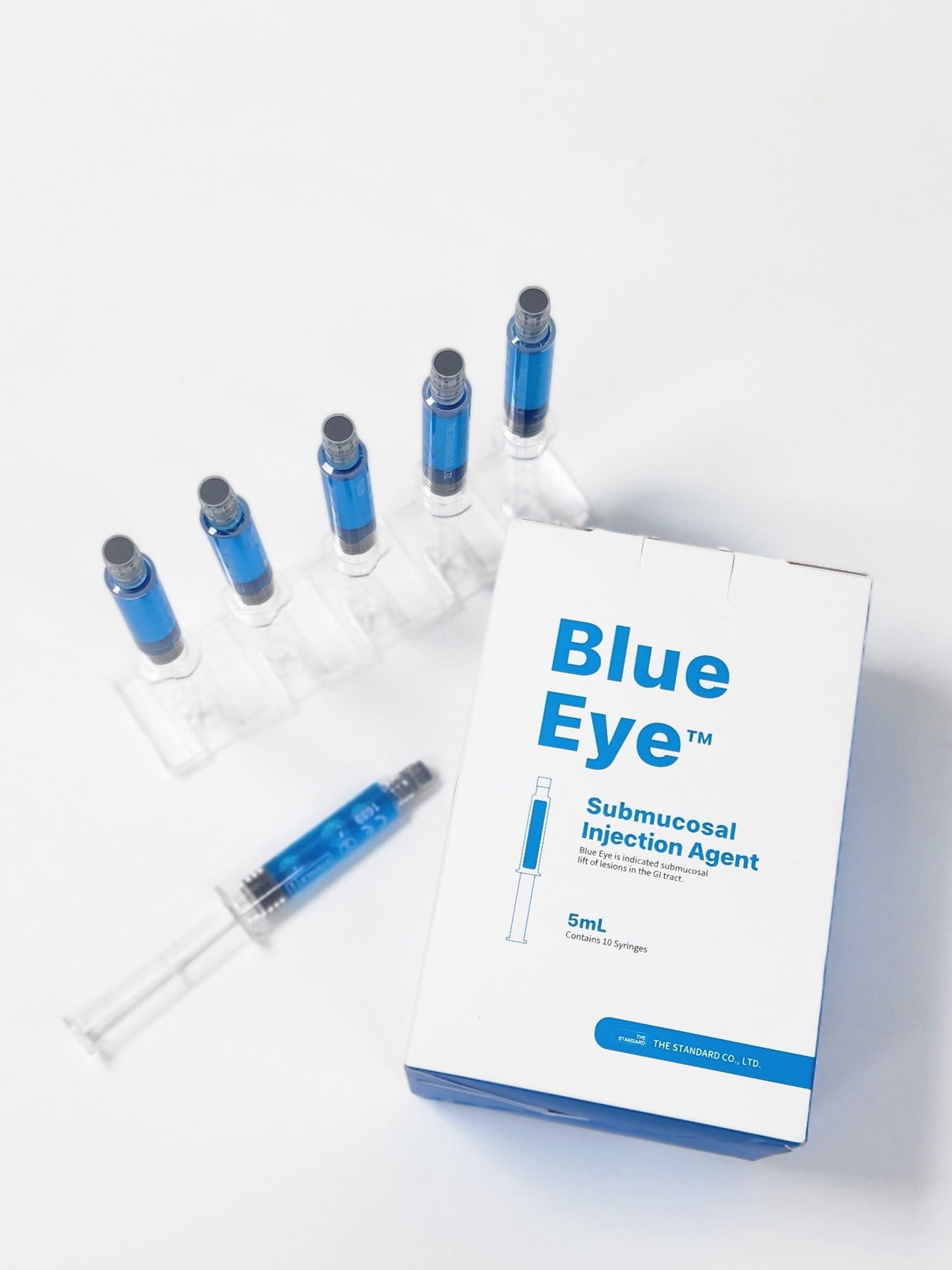
Blue Eye is a submucosal injection agent used in the gastrointestinal system during endoscopic procedures to lift polyp adenomas and early-stage cancers prior to their removal with a snare or endoscopic device.
Blue Eye facilitates endoscopic resection procedures during examinations of both the upper and lower gastrointestinal tract, including the esophagus and stomach.
Safe from contamination
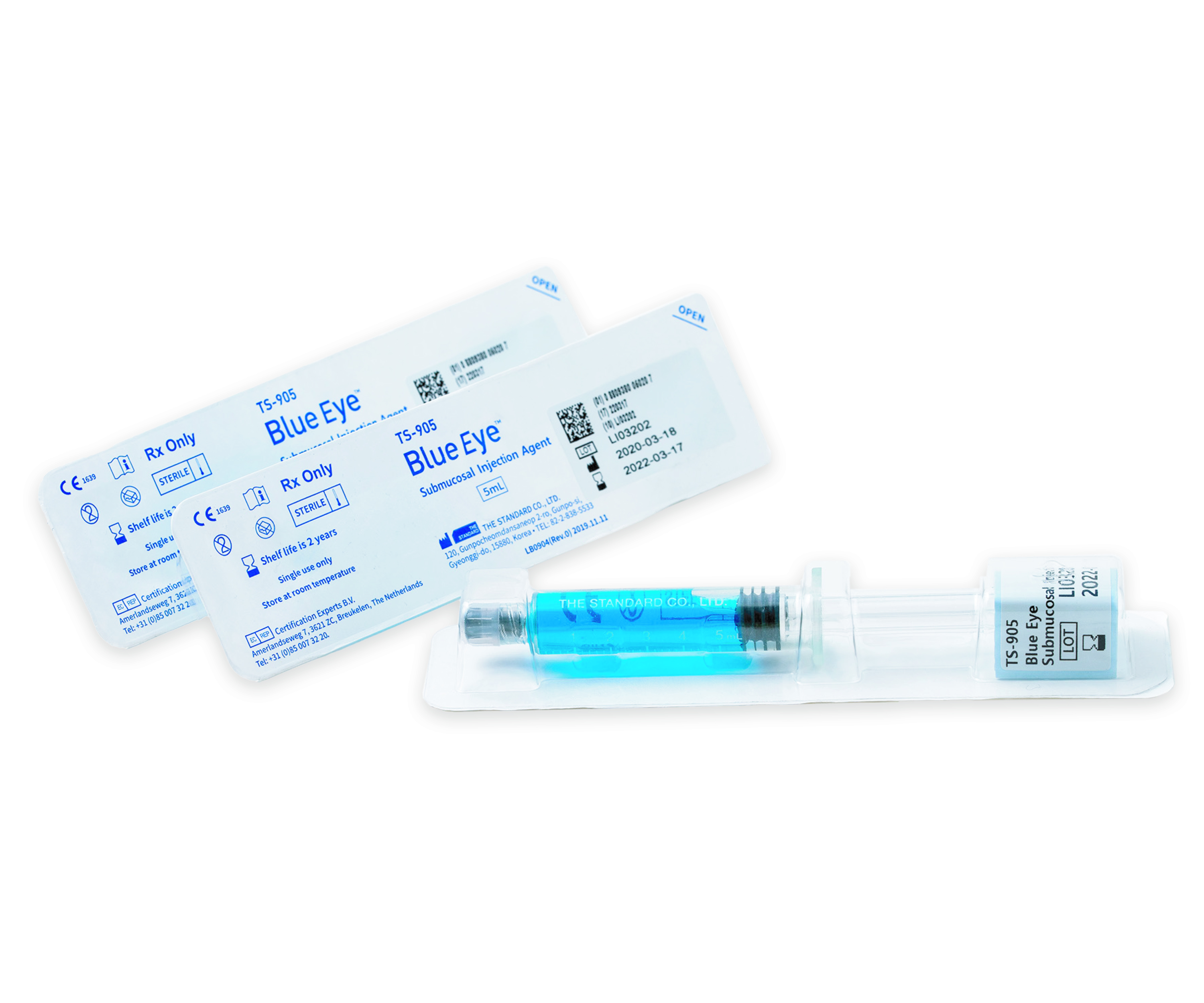
Blue Eye™ is packaged in a ready-to-use syringe containing a steam-sterilized solution that connects directly to the injection needle with a Luer lock, eliminating the risk of cross-contamination by preventing air exposure.
Easy to use
Unlike conventional ampoule products, Blue Eye™ can be directly connected to the injection needle, making it easy to use.
Biocompatible
Blue Eye™ is biocompatible and contains hyaluronic acid, a biopolymer naturally found in human dermis.
Sodium Hyaluronate
Sodium hyaluronate is the sodium salt of hyaluronic acid, a glycosaminoglycan found in various human connective tissues.
Effective
Blue Eye™ provides mucosal elevation that lasts twice as long as normal saline, reducing the total procedure time and side effects such as perforation, thereby increasing the success rate of the endoscopic procedure.
Advantages of Blue Eye™
Important Safety Information
Warnings and Precautions
The safety of Blue Eye™ in pregnant or breastfeeding women or in children under 18 years of age has not been established.
The endoscopist administering Blue Eye™ must be experienced in the application technique.
Adverse Reactions
Rarely, local bleeding and/or inflammatory reactions may occur, which may or may not be associated with Blue Eye™.
Contraindications
Patients with known hypersensitivity to any of the components in Blue Eye™. Please refer to the Instructions for Use for complete Important Safety Information.